The GOZILA Study is a research project that aims to improve the treatment of patients with cancer through liquid biopsy.




Why was the GOZILA Study started?
- The GOZILA study is a clinical research.
- Clinical research refers to studies conducted with the cooperation of patients to improve disease diagnosis and treatment methods.
-
This research was carried out through partnerships between patients, the public, and researchers.
For more information, please see the PPI page
※The official name of "GOZILA" in the GOZILA Study in Japanese is "Study on Liquid Biopsy in Patients with Gastrointestinal and Abdominal Malignancies Including Colorectal Cancer".
GI-SCREEN(now MONSTAR-SCREEN)
It was known that each patient's cancer shows different gene variants, and treatments according to these gene variants (targeted therapy) can be highly effective.
In Japan, however, there has not been a
sufficient system for patients with cancer to get genetic testing and receive corresponding treatments.
That is why we launched the SCRUM-Japan GI-SCREEN in 2015 to enable patients examine many cancer genes at once and receive treatments according to their results.
How can we make genetic testing more accessible...?
In SCRUM-Japan GI-SCREEN, the genetic testing was conducted using tumor tissue samples from obtained through surgery or other procedures.
However, there were several problems with this approach.
※Genetic testing that primarily examine somatic gene variants.
- Because of the time it takes to obtain the test results after the tissue is removed, there is a delay in providing the results to the patient.
- Obtaining tumor tissue requires surgery or other procedures, which puts a physical burden on patients.
-
It has now been known that when cancer is treated become more resistant to treatment.
However, with the tissue that was previously collected, we cannot know about these subsequent changes.
To solve these problems, we need to consider a new testing method.

How do we examine genes through Blood testing?
At that time, we came across a new technology called "liquid biopsy”.
- Liquid biopsy is a technology that examines cancer DNA in the bloodstream(called ctDNA) to detect gene variants.
- If this method becomes available, patients may be able to choose their treatment quickly without physical burdens based on the latest gene information.
Therefore, we launched the GOZILA Study in 2018, a study to find appropriate treatments for patients with cancer by performing liquid biopsies.
- The important goal of the GOZILA Study is to deliver suitable treatment for each patient.
-
In this project, we use liquid biopsy (ctDNAtesting) to examine in detail the gene variants
associated with cancer. - Based on the results, we aim to find treatment suitable for each patient.


Our Research Process
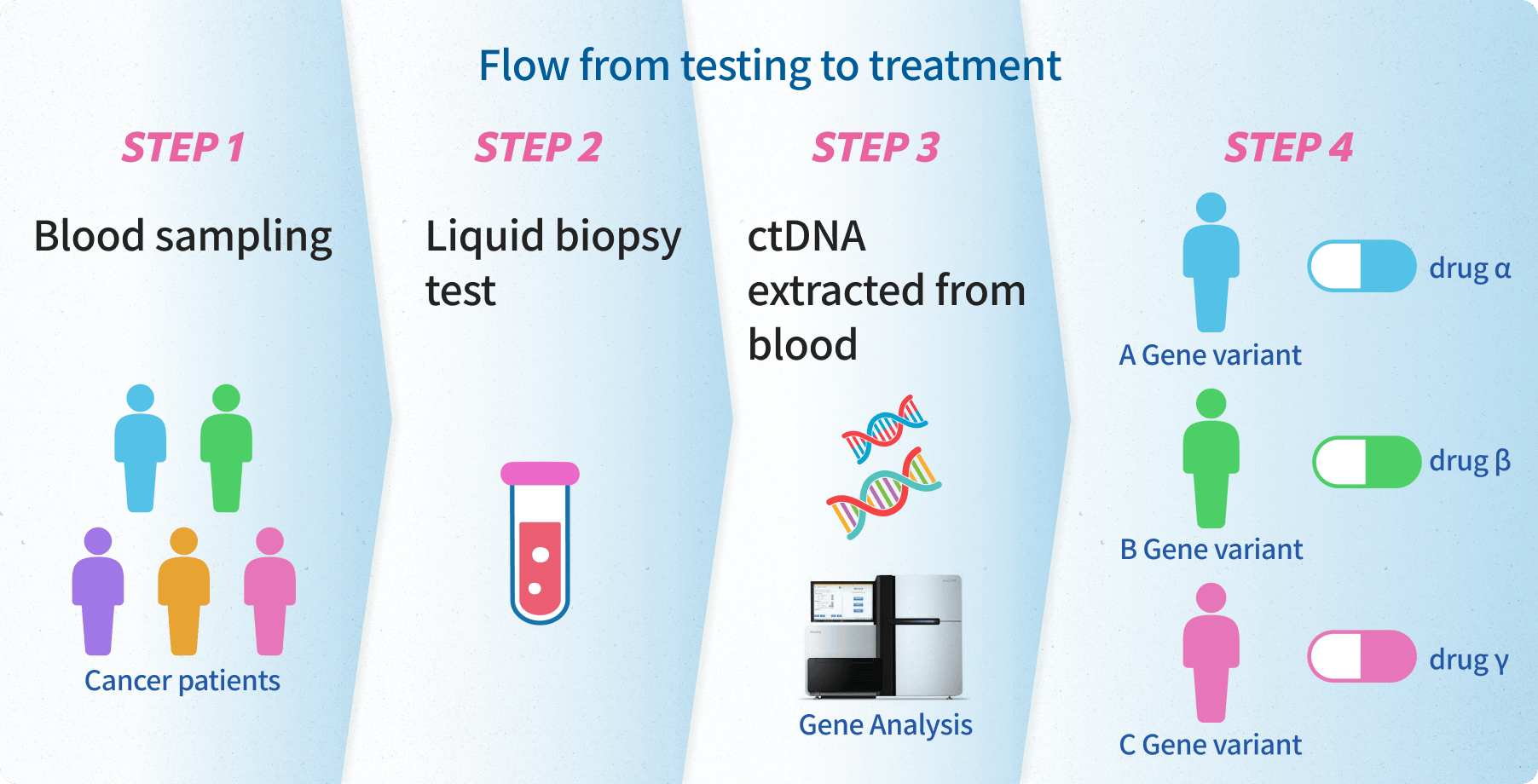
In the GOZILA study, we conducted genetic testing using blood samples from patients
who are currently receiving anticancer drugs or are scheduled to receive them.
- We examine 74 different types of genes at once using blood samples.
- When the test results come back, the doctors participating in the research will carefully consider the information and propose the treatment method that they think will be suitable for the patient.
Furthermore, this project explores possibilities for new treatments.
Even for drugs that cannot be used in regular insurance-covered medical care, we made them available through
clinical trials.
Treatment using liquid biopsy testing (Visual)

The patients who participated
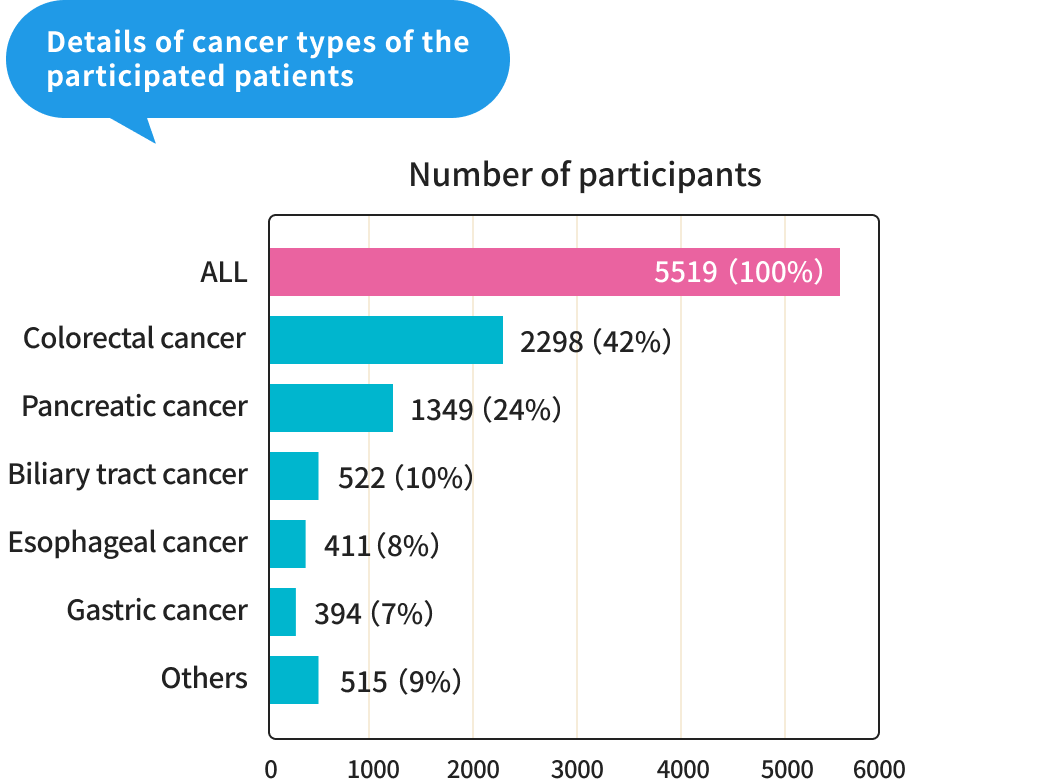
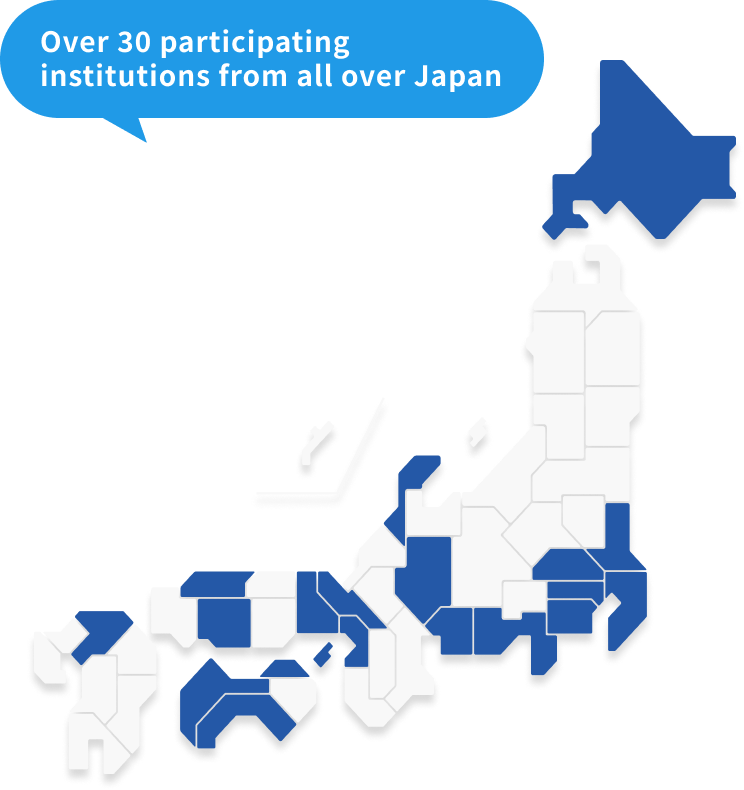
From 2018 to the end of July 2024, we enrolled 5,519 patients from approximately 30 facilities nationwide (enrollment has now ended).
We would like to express our sincere gratitude to the patients, their families, and all those involved who cooperated with this study.
in his 40s
Participant Interviews
※ His partner was also present during the interview.
I don't remember much about joining the research because I had so much else going on at the time. But I think my feeling was 'Please, go ahead and use my blood if it can help.' I didn't even know it was
called GOZILA.
Since the research only involved blood draws, it wasn't burdensome at all. While I don't have specific suggestions for improvement, I think it would be good if more people knew about this kind of
research being conducted. To other patients, I want to say that I'm doing my best too, so keep fighting! And to the research doctors -I have high hopes for you!
in her 50s
Participant Interviews
I joined hoping that better treatments would be developed. I thought if I could help others who are suffering, that would be good.
The liquid biopsy test was just a blood draw, so there was no burden or worry. While my feelings have their ups and downs, I can think more positively knowing there's an option to live while coexisting
with cancer as it gets smaller.
I didn't find the research explanation documents particularly difficult. I think those who can participate in the research probably should.
After receiving the liquid biopsy, I became more interested in learning about my own genes.
in his 50s
Participant: Female in her
50s partner of Mr.K
Interview from a family member's perspective
I still think it was really great that my wife matched with both GOZILA and a clinical trial. For our family, our primary hope was for effective treatment. And while it wasn't as big a reason, we also
thought it might help others in the future.
Although there's still no cure, I hope I can also contribute to research myself someday, as one way to take a positive approach.
Throughout the treatment, we discovered and learned a lot as a family. I believe it's important to stay positive and cherish the valuable time we spend together as a family.


Research results
Thanks to the participants, the GOZILA Study has progressed, and we have been able to produce many research results. It is hoped that the GOZILA Study will enable us to provide better possible medical care to even more patients.
Lay Summary
The Lay Summary is a summary of the content and results of the study for patients, their families and the public, making it easier for anyone to understand the medical journal, which was written for experts.
The GOZILA Study Lay Summary was created in consultation with patients.
The GOZILA Study Lay Summary(Japanese)
- Related Information : SCRUM-Japan GOZILA Confirms Extended Survival with Personalized Cancer Treatment in 4,000+ Patients Study
- Link:SCRUM-Japan GOZILA Confirms Extended Survival with Personalized Cancer Treatment in 4,000+ Patients Study | National Cancer Center Japan
Approval of new treatments and tests
(regulatory approval)
From the study and its related research and clinical trials, 2 therapeutic drugs and 4 diagnostics tests (with 9 indications) have received regulatory approval and are now available under insurance reimbursement.
instability
instability
gene mutation
mutation
number variation
We will continue to pursue research
that benefits patients and the public

supported the GOZILA Study

participating in the GOZILA Study