
What is “PPI”?
Patient and Public Involvement (PPI)
Patient and public involvement in medical research and clinical trials refers to the process of medical research and clinical trials in which researchers refer to the findings of patients and the public.
There are various steps in research, and the opinions and ideas of patients and the public are useful depending on the content of the research.
How is PPI implemented?
As shown in the illustration below, the “PPI” aims to develop better medical science through the exchange of opinions between researchers, patients and the public.
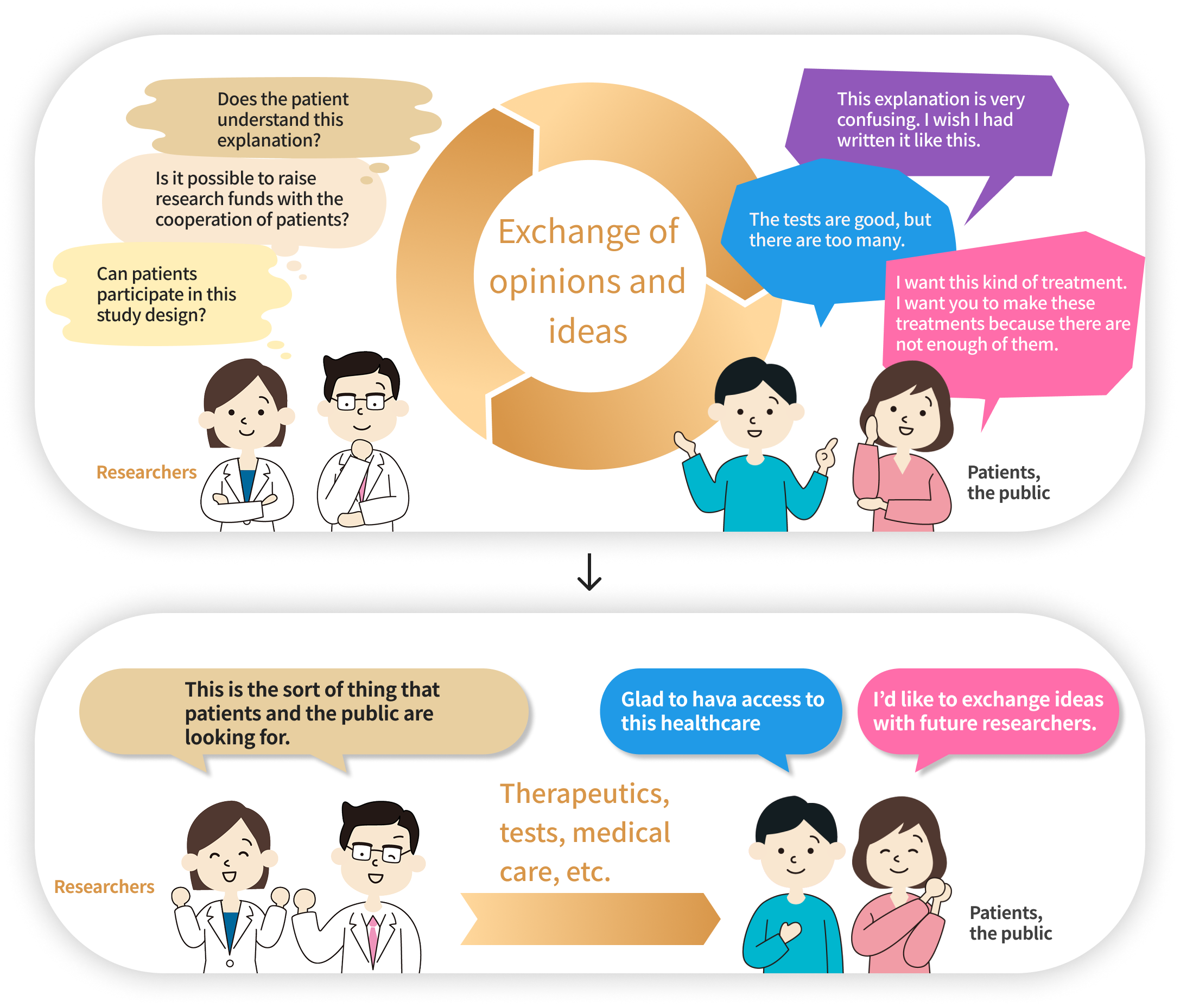
How is the “GOZILA Study” implementing PPI?
GOZILA Study
The GOZILA Study is a clinical study evaluating whether a blood test called liquid biopsy is useful in detecting gene variants in cancer.
The GOZILA Study was designed by researchers, and patients participated in the study.
-
Documentation Improvement
-
Patient and Public Feedback
PPI of the GOZILA Study ❶
Document Improvement


In the GOZILA study, we discussed whether the “explanatory document” was difficult to understand for patients and whether there was any information lacking.
As a result, we received a total of
48 comments from three representatives
from patients and the public.
Of the 48 comments we received, 20 were specific wording corrections, additions or suggestions, half of which were “it’s hard to
understand because of the terminology”.
The next 18 comments was “Need more details to understand”, and the next 5 comments were about information disclosure: “Please
describe where I can find additional information”.


PPI of the GOZILA Study ❷
Patient and Public Feedback

Patients, the public and researchers were brought together for discussion and regular exchange of opinions and ideas was organized. As a result, we received a lot of feedback from everyone who attended.
I would like to use it for cancer screening.
Please incorporate in hematologic oncology.
Detecting early-stage cancers is important for a high-risk population.
I want to know the stage and type of cancer
in the screening.
Hope to know how impactful the side effects are, and how effective they are.
Collaboration and data sharing around the world for development.
I wish I could have screening at home or at a local clinic.
It would be nice to know what the next treatment option is.
It would be a cost-saving alternative to CT, PET and MRI with follow-up.
It would be good if people who are genetically predisposed to cancer could also be tested to resolve their concerns.
Good for cancers which are difficult to find.
I would like to see progress made in areas where genetic analysis is not yet underway.
Liquid biopsies are less physically and psychologically burdensome than other tests.
Opinions such as that liquid biopsy is a “No burden test” “hope to know the side effects,” “good to test at a nearby clinic,” “Useful for people with hereditary cancers” were comments that researchers could not focus on yet.
From gathering opinions to new research.
The opinions and ideas we have received have been very helpful for the researcher of GOZILA Study and the related studies.
In fact, based on these findings, a new research plan is beginning to move forward using liquid biopsies.

Members of the
SCRUM-Japan MONSTAR-SCREEN PPI Committee
As of October 1, 2024
SCRUM-Japan MONSTAR-SCREEN PPI Committee
- Japan Federation of Cancer Patient Groups
- Shinsuke Amano, Chair
- Kimie Sakurai
- Naomi Sakurai
- Yoshiyuki Majima
- Japan Pharmaceutical Manufacturers Association
- Masayo Miyata(Janssen Pharma)
- Inaha Okuda(GlaxoSmithKline)
- National Cancer Center Hospital East
- Takayuki Yoshino, Physician, Researcher
- Hideaki Bando, Physician, Researcher
- Yoshiaki Nakamura, Physician, Researcher
- Takao Fujisawa, Physician, Researcher
- Mitsuho Imai, Physician, Researcher
- Yu Komura, Study manager
- Maiko Takakusa, Study manager
- Mihoko Yuasa, Study manager
- Mizuho Kikuchi, Study manager
- Yui Ogata, Study manager
- Yukie Kimura, Clinical Research Coordinator
- Kiyoko Adachi, Clinical Research Coordinator
- Sayuri Nochi, Clinical Research Coordinator
- Sakie Takasu, Clinical Research Coordinator
- Izumi Miki, Study manager
This website was created with research funding from “Project Future” from Relay for Life Japan
"Building a Foundation for Patient and Public Participatory Clinical Research in the SCRUM-Japan Study." 2019 Izumi Miki





